Fractional distillation is a process by which components in a chemical mixture are separated into different parts called fractions according to their different boiling points. Similar to continuous shell still the fractional distillation process is made up of several stills linked together in series.
Purification Fractional Distillation
It is useful for separating ethanol from a mixture of ethanol and water and for separating crude oil into.

Process of fractional distillation. Fractional distillation of crude oil Fractional distillation separates a mixture into a number of different parts called fractions. B The condensed liquid of the fraction is collected in a tray. Fractional distillation is a type of distillation which involves the separation of miscible liquids.
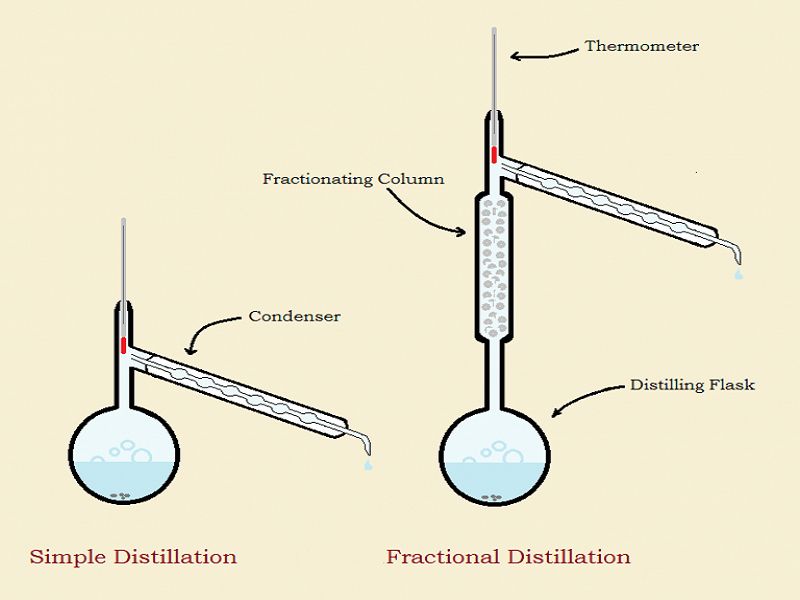
As the feed is partially vaporised in the first still the vapors rise travel through the overhead line and come in contact with the. An assembled fractional distillation apparatus is shown in Figure 543 using glass beads in the fractionating column. Fractional distillation is the process of separating crude oil into groups of hydrocarbons with similar numbers of carbon atoms.
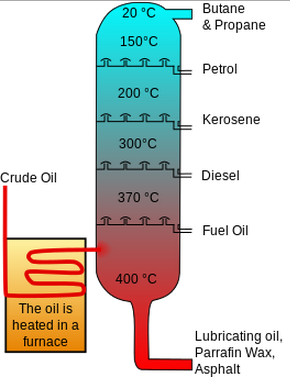
Crude oil distillers separate crude oil into fractions for subsequent processing in such units as catalytic reformers cracking units alkylation units or cokers. Fractional distillation separates miscible liquids that have different boiling points. In these refineries the crude oil is pumped through pipes into the fractional distillation tower.
The basic steps to distillation are. In turn each of. We call these groups of hydrocarbons fractions.
The principle behind this process tells us that all components of the substances are boiling which comes from variations of temperature. Fractional Distillation Procedure. From this the fractions gets separated from one another.
Fractional distillation is used to purify chemicals and to separate mixtures to obtain their components. This process is now known as fractional distillation. The Process The process begins by drilling into the reservoir rocks the ground and extracting the crude oil.
D 1 This is how the distillation process in the fractionator works. Fractions are defined as the many different components of hydrocarbon from crude oil. This is very simple method of separation if boiling point difference is.
E Most of the fractions in the crude oil evaporate. As the liquid heats components with the lower boiling points will begin to vaporize and rise through the column. The primary process for separating the hydrocarbon components of crude oil is fractional distillation.
It is assumed that readers have previously performed a simple distillation so in this section are described differences between simple and fractional distillation. Fractional distillation is the process of separating a mixture into its different components. A tall fractionating column is fitted above the mixture with.
Fractional Distillation or Distillation or Fractionation is the process of separation of components from a product or mixture Crude Oil by heating the mixture to a temperature at which several fractions of the compound will vaporize. Other columns may be substituted. Once extracted it is sent to one of the many refineries around the world.
As vapor rises in the distillation. The main difference is that all the liquid condensate is returned to the upstream still. The process involves repeated distillations and condensations and the mixture is usually separated into component parts.
Add heat to a liquid mixture with two or more main substances. For example a water and ethanol mixture. In simple distillation we heat the mixture at normal pressure and separate the low boiling components from high boiling components As like fraction distillation this type not required any type of packing and reflux.
The separation happens when the mixture is heated at a certain temperature where fractions of the mixture start to vaporize. The difference is that simple distillation does this process once while fractional distillation repeats the process several times within the same system. F The condensed liquid flows out of the fractionator through a pipe from the tray.
C When a fraction in the vapour cools to its own boiling point it condenses. Its similar to simple distillation in that it uses heat evaporation and cooling condensation to separate substances.
 Process Of Fractional Distillation
Process Of Fractional Distillation

 Fractional Distillation Is Useful For Separating A Mixture Of Substances
Fractional Distillation Is Useful For Separating A Mixture Of Substances
 Diagram Essential Oil Distillation Diagram Full Version Hd Quality Distillation Diagram Beefdiagram Andreavellani It
Diagram Essential Oil Distillation Diagram Full Version Hd Quality Distillation Diagram Beefdiagram Andreavellani It
 6 Fractional Distillation Examples In Everyday Life Studiousguy
6 Fractional Distillation Examples In Everyday Life Studiousguy
 Fractional Distillation Wikipedia
Fractional Distillation Wikipedia
 Fractional Distillation Energy Education
Fractional Distillation Energy Education
 Fractional Distillation Wikipedia
Fractional Distillation Wikipedia
A What Is Fractional Distillation List The Two Conditions Essential For Using This As A Method Of Separation Of Components Of A Mixture B Draw A Label Chemistry Topperlearning Com 1rx1w9xtt