Citrus fruitssuch as grapefruitcontain high amounts of citric acid a common organic acid. To resolve these problems there is a more advanced acid-base theory.
 Lewis Acids And Bases Chemistry Steps
Lewis Acids And Bases Chemistry Steps
The first modern definition of acids and bases in molecular terms was devised by Svante Arrhenius.

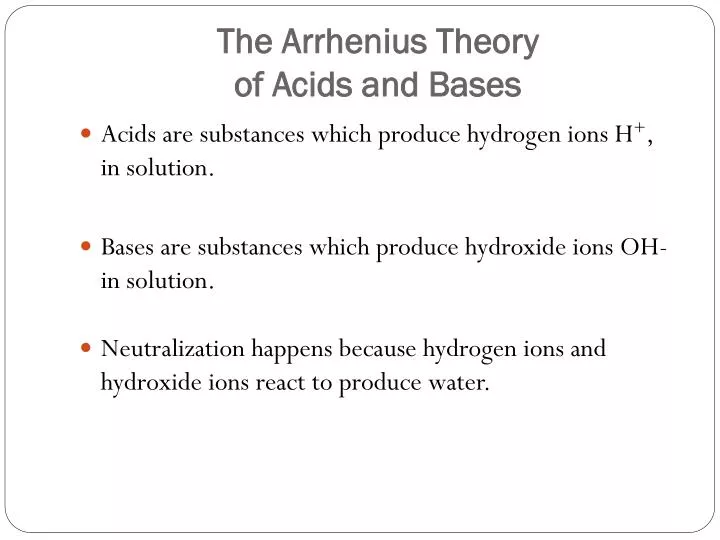
Arrhenius acid base theory. There are many well known bases such as ammonia NH 3 that do not contain the hydroxide ion. This definition identified most of the substances which were considered to be acids at that time. Neutralisation happens because hydrogen ions and hydroxide ions react to produce water.
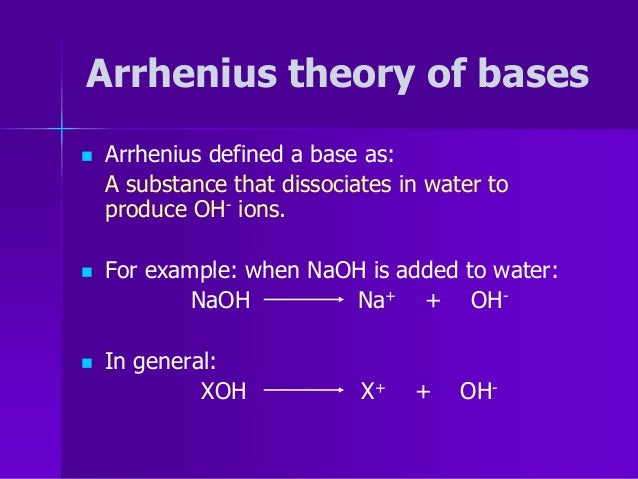
We will discuss THREE of these theories. An Arrhenius base is a compound that increases the OH ion concentration in aqueous solution. An Arrhenius acid is a compound that increases the H ion concentration and an Arrhenius base is a compound that increases the OH ion concentration in aqueous solution.
He defined an acid as a substance which was capable of dissociating in water solution to produce hydrogen ions. According to Arrhenius theory acids are the compound that increases the concentration of H or proton in aqueous solution. The Arrhenius acid base theory was introduced in the late 19th century.
However the ChemTeam thinks the actual first statement of the theory is in his 1887 publication concerning the electrolytic dissociation theory. The theory does not consider the role of solvent in deciding the nature of acid and base. Similarly the theory is unable to explain the basic properties of Na 2 CO 3 amines pyridine NH 3 as they cannot be represented by the formula BOH.
The Arrhenius Theory of acids and bases. Furthermore acid-base reactions are observed in solutions that do not contain water. - Several scientific theories exist that define acid-base chemistry.
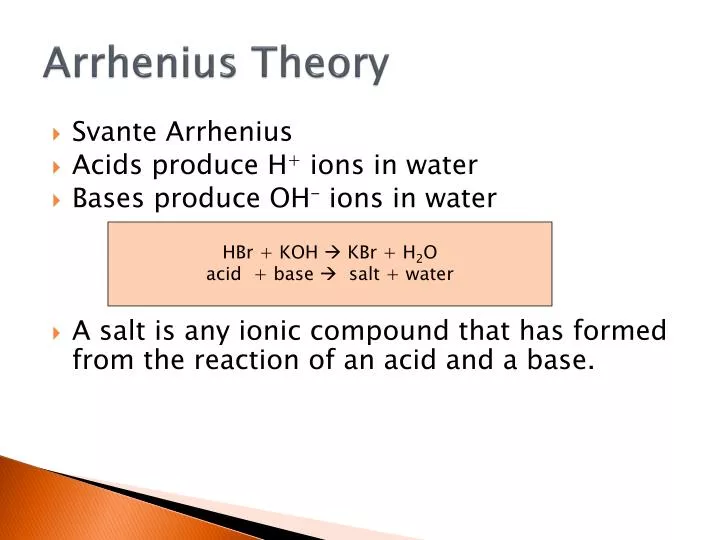
Arrhenius acid and base theory. Hydrochloric acid is neutralised by both sodium hydroxide solution and ammonia solution. As defined by Arrhenius.
THREE ACID-BASE THEORIES Arrhenius theory. Defines acids and bases according to Arrhenius theory and the neutralization reaction. The Arrhenius Theory The first modern approach to acid-base chemistry was by Arrhenius in 1887.
Bases are substances which produce hydroxide ions in solution. A Swedish scientist Svante Arrhenius in the year 1884 proposed acid and base as the two classifications of compounds. It was used to provide a modern definition of acids and bases and followed from Arrheniuss work with Friedrich Wilhelm Ostwald in establishing the presence of ions in aqueous solution in 1884.
This way of defining acids and bases works well for aqueous solutions but acid and base properties are observed in other settings. All Arrhenius acids and bases are Brønsted-Lowry acids and bases as well. One would be a hydrogen ion.
A substance is classified as a base if it produces hydroxide ions OH- in water. The Arrhenius theory of acids and bases was originally proposed by the Swedish chemist Svante Arrhenius in 1884. He suggested classifying certain compounds as acids or bases based on what kind of ions formed when the compound was added to water.
Ionic compounds of the OH ion are classic Arrhenius bases. Arrhenius theory theory introduced in 1887 by the Swedish scientist Svante Arrhenius that acids are substances that dissociate in water to yield electrically charged atoms or molecules called ions one of which is a hydrogen ion H and that bases ionize in water to yield hydroxide ions OH. It was the first modern approach to acid-base concept.
Bases on the other hand would yield hydroxide ions. The Arrhenius definition of acid-base reactions is a development of the hydrogen theory of acids. Created by Svante Arrhenius the idea was that acids were a substance that would disassociate in water to yield ions that were electrically charged.
Thus HCl is strong acid when dissolved in water but it is weak acid when dissolved in benzene. - Typically the newer theories include MORE chemicals under the umbrella of acid-base chemistry. Limitations of the theory.
Acids are substances which produce hydrogen ions in solution. The ChemTeam is working on finding out. The Arrhenius acid-base theory was proposed by Swedish Svante Arrhenius.
However the Arrhenius theory is not without flaws. A hydrogen theory of acids it followed from his 1884 work with Friedrich Wilhelm Ostwald in establishing the presence of ions in aqueous solution and led to Arrhenius receiving the Nobel Prize in Chemistry in 1903. Example 1 Identify each compound as an Arrhenius acid an Arrhenius base or neither.
Also discusses the difference between strong and weak acids and how to. A Brønsted-Lowry base is a proton acceptor. - These theories differ in the way that acids bases and their associated reactions are defined.
This theory is quite simple and useful. Arrhenius Acid and Base Definition. Here we will discuss Arrhenius theory of acids in details.
The Acid Base Theory Arrhenius published two articles on acids and bases one in 1894 and the other in 1899. A Brønsted-Lowry acid is a proton donor. The Arrhenius acid-base concept classifies a substance as an acid if it produces hydrogen ions H or hydronium ions in water.
 Bronsted Lowry Theory Definition Examples And Limitations
Bronsted Lowry Theory Definition Examples And Limitations
 Arrhenius Acid Base Theory Youtube
Arrhenius Acid Base Theory Youtube
 Ppt Arrhenius Theory Powerpoint Presentation Free Download Id 2276434
Ppt Arrhenius Theory Powerpoint Presentation Free Download Id 2276434
 Ppt The Arrhenius Theory Of Acids And Bases Powerpoint Presentation Free Download Id 3092912
Ppt The Arrhenius Theory Of Acids And Bases Powerpoint Presentation Free Download Id 3092912
 Acids Bases Salts The Arrhenius Theory Of Acids And Bases Ppt Download
Acids Bases Salts The Arrhenius Theory Of Acids And Bases Ppt Download
 Wcln The Arrhenius Theory Of Acids Chemistry Youtube
Wcln The Arrhenius Theory Of Acids Chemistry Youtube
Definitions Of Arrhenius Bronsted Lowry And Lewis Acids And Bases In Organic Chemistry